Research Question: Does the amount of acetone affect the length of the “black snake”
IV: The amount of Acetone
DV: The length of the snake
Controlled Variables: Amount of sugar, sand, baking soda.
Hypothesis: We believe that the more acetone we put in the longer the “black snake” will be. We believe so because the acetone is highly flammable which could have more reaction on the snake. This meaning, that the fire will last longer, hence making the snake longer.
Data table:
| Observations | Amount of acetone (ml) | Length of snake (cm) |
| We think we took too long to light it up and the liquid dried out | 5ml | 0cm |
| We put the liquid in one place and lit it up as soon as possible though a tiny reaction, almost nothing happened | 5ml | 0cm |
| The reaction came really quick. I’m really happy! | 20ml | 10cm (height) 12cm (length) |
| We didn’t leave any space for the fire to reach the acetone. | 15ml | 4cm (height) 9cm (length) |
| The experiment went better than the others. Though we left a crevasse that was too big so the base wasn’t as sturdy. | 25ml | 9cm (height) 17cm (length) |
Graph (scatter plot with line of best fit.) 
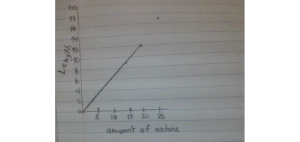
Discussion: The experiments started of with a couple of failures. Though as we raised the amount of acetone and used the fire from the gas tank on top of that, our results were amazing.The whole experiment worked. Adding the extra fire from the gas tank was a great help as well. The growth of the snake exceeded our expectations in the end. Next time we should try to find a way to make it burn on it’s own and have a solid base.
Conclusion: Based on my data, I can conclude that the higher the amount of acetone is, the longer the snake grew. Even though on the 25 ml of acetone snake had a bigger crevasse than needed so the fire reach it and it could burn on it’s own. Even though the base wasn’t as sturdy in total it grew the most(9 cm in height and 17cm in length). The experiments that only got 5 ml of acetone didn’t grow at all because their wasn’t enough heat for the reaction to happen.



